A diagnosis of Lyme Disease should be based on symptoms, physical findings and exposure to infected ticks.
A negative blood test does not mean you do not have Lyme Disease.
There are many reasons why someone who has Lyme Disease may have a negative test result1:
- Length of infection (during the first 4-6 weeks of infection, most people have not yet developed the antibody response that the test measures2)
- Low or nonexistent antibody levels caused by early treatment with antibiotics
- A co-infection may be present in the blood and not the Lyme bacteria
- Infection may be caused by a strain of Borrelia burgdorferi (Lyme Bacteria) that is not covered in testing
- Immune system may be suppressed
The most commonly used two-tiered testing for Lyme Disease are:
- ELISA (enzyme-linked immunosorbent assay)
- Western blot
Unfortunately, numerous studies have shown that the ELISA test is not sensitive enough for screening and often misses the infection. In a study3, published by Raphael B. Stricker and Lorraine Johnson from the International Lyme and Associated Diseases Society (ILADS), it was found that “the sensitivity of the two-tier approach in Lyme Disease patients tested at least 4 to 6 weeks after infection is only 44% to 56%, which is inadequate for a clinical diagnostic test” and that “testing for Lyme Disease remains problematic.” Holly Ahern, Associate Professor of Microbiology at SUNY Adirondack, stated, “We’re missing easily half of the cases of Lyme Disease. If you flipped a coin, it’s about as reliable.”
Because of the unreliability of the ELISA screening, Lyme Disease organizations and many Lyme-literate doctors , like Joseph J. Burrascano Jr., MD, suggest that patients skip the ELISA and specifically request the Western Blot. ILADS suggests that the Western Blot screening “should be performed by a laboratory that reads and reports all of the bands related to Borrelia Burgdorferi4.”
Other tests that are used to diagnose Lyme Disease are:
- Polymerase chain reaction (PCR) – a highly accurate test when the Lyme DNA is detected, however, it does produce many false negatives because Lyme bacteria are sparse and may not be in the sample tested.
- Antigen capture – a detection test that looks for a unique Lyme protein in body fluids (e.g. blood, urine, joint fluid). People who test negative on other indirect tests may test positive on this test.
- CD57 – a test that measures the levels of a subset of Natural Killer cells and is used as a marker to measure the level of active infection.
Another extremely important component in the treatment of Lyme Disease, is whether or not a co-infection is present. An increasing number of ticks have been found to carry and transmit other infectious agents such as Babesia, Ehrlichia and Bartonella (click here for more on co-infections). Tell your doctor that you want to be tested for these co-infections.
To date, there is no test available that can positively rule out Lyme Disease. However, the following is a list of labs that specialize in the testing of tick-borne infections. These labs are licensed and monitored by the Centers for Medicare and Medicaid Services (CMM), part of the Department of Health and Human Services, through the Clinical Laboratory Improvement Ammendments (CLIA). Several labs have also received accreditation by the College of American Pathologists (CAP) whose goal is to improve patient safety by ensuring laboratories meet or exceed regulatory requirements.
- Advanced Laboratory Services (CLIA #39D1102884)
- Clongen Laboratories (CLIA #21D1032144)
- Fry Laboratories (CLIA #03D1026968)
- Galaxy Diagnostics (CLIA #34D2027997)
- IGeneX (CLIA #05D0643914)
- Imugen (CLIA #22D0650196; CAP Accredited)
- Immunosciences Lab (CLIA #05D0642471; CAP Accredited)
- LabCorp (CLIA #34D0655205)
- Medical Diagnostics Laboratories (CLIA #31D0938156; CAP Accredited)
- Stony Brook School of Medicine (CLIA #33D0654233)
Disclaimer: The inclusion and/or omittance of a laboratory shall not be construed as a particular bias toward any organization by the Wayne County Lyme Disease Task Force. This information is intended solely as a resource tool to assist you in finding a laboratory that provides tick-borne illness testing.
TWO-TIERED LAB TESTS MISS MORE THAN 50% OF THE CASES OF LYME DISEASE5
Lyme disease diagnosis and treatment: Lessons from the AIDS epidemic
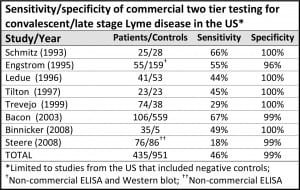
Johnson L, Stricker RB. Minerva Med. 2010; 101: 419-25The most commonly used tests to diagnose Lyme Disease are the ELISA (enzyme-linked immunosorbent assay) and the Western Blot. However, studies find that these tests are not very accurate and may miss a diagnosis in more than 50% of patients.
The most common tests for Lyme disease measure the patient’s antibody response to infection. When your body is invaded by the Lyme spirochetes, your immune system makes antibodies to fight the infection. Tests that measure antibody levels are indirect tests. They measure the body’s immune response to infection rather than the actual presence of bacteria.
The Centers for Disease Control recommends two-tiered antibody testing for Lyme disease. The first step is an ELISA test. If positive, it is followed up with a Western blot. The CDC recommends two-tiered tests for HIV/AIDS as well. However, the AIDS two-tiered tests have a sensitivity of 99.5%, meaning that they catch nearly every case. Unfortunately, in Lyme disease the two-tiered approach has a sensitivity of less than 50%, meaning that it misses more than half of the cases.
(The chart above is adapted from Stricker, R. and Johnson, L., Lyme disease diagnosis and treatment: Lessons from the AIDS epidemic. Minerva Med. 2010; 101: 419-25.)
There are a number of reasons for the difference. The most important one is that the current ELISA tests are not sensitive enough for screening. Studies by the College of American Pathologists (CAP) concluded that the currently available ELISA tests for Lyme do not have adequate sensitivity to meet the two-tiered approach recommended by the CDC for surveillance.
In his research, Dr. Sam Donta has observed that 52% of patients with chronic Lyme disease are found negative by ELISA but positive by Western blot. This corresponds with the published findings of LymeDisease.org’s survey of over 5,000 patients with chronic Lyme disease. The survey found that 30% of patients were diagnosed by two-tiered serology and 24% were diagnosed by Western blot alone.
1“Choosing A Lyme Disease Test.” Daniel Cameron MD. Web. 29 Feb. 2016
2“Lyme Disease Is a Clinical Diagnosis, Based on Your Medical History, Symptoms and Exposure to Ticks.” LymeDisease.org. Web. 01 Mar. 2016.
3Stricker, Raphael B., and Lorraine Johnson. “Lyme Disease: The next Decade.” Infection and Drug Resistance. Dove Medical Press. Web. 01 Mar. 2016
4“Basic Information about Lyme Disease.” ILADS.org. ILADS. Web. 01 Mar. 2016.
5“Two-Tiered Lab Tests Miss More Than 50% Of The Cases Of Lyme Disease.” Lymedisease.org. Web. 29 Feb. 2016